When it comes to the distinctive aroma of cannabis, most of the discussion is about terpenes, a class of molecules that makes up the greater part of the volatiles found in mature flower. Terpenes such as D-limonene and alpha- and beta-pinene no doubt contribute to some of the citrus and pungent aromas typical of certain cultivars. But it’s always been clear to me that terpenes are unlikely to account for the skunky, funky, ganja-like character common to all cannabis cultivars. Why? Because skunky notes strongly imply the presence of a sulfur-containing aroma molecules—you can’t get to skunky with terpenes alone. No mystery: just basic fragrance chemistry.
The existence of sulfur-containing molecules in weed has long been suspected, but never scientifically confirmed. There are a couple of reasons for this. First, sulfur-containing molecules often have high aroma impact—they can be perceived at extremely low concentrations. Anyone looking for them must use extraordinarily sensitive analytical methods. Second, these compounds emerge from the gas chromatograph in a different portion of the volatile spectrum if you will; if you’re focused on terpenes you’ll miss them.
On a practical level, it seemed likely that sulfur-containing molecules are the basis for the odor complaints frequently lodged against cannabis cultivation facilities. Would the neighbors really get angry about some pine-like or citrus scents wafting down the block? Nah. But if the grow house is releasing a sulfur-containing thiol of some type, their response would make total sense.
There have been hints that a sulfur-containing molecule might underlie the basic cannabis aroma. In 2012, researchers in Spain identified an uncommon thiol (3-methyl-2-butene-1-thiol or 321 MBT) in a Spanish red wine made from Prieto Picudo grapes. Sensory panelists described 321 MBT as having a “marihuana-like, rubber-like, and beer-like” aroma (San-Juan, et al., 2012).
Earlier this year a mixed industry-academic team announced by press release that 321 MBT is the culprit behind cannabis odor complaints. Intriguing, but not definitive.
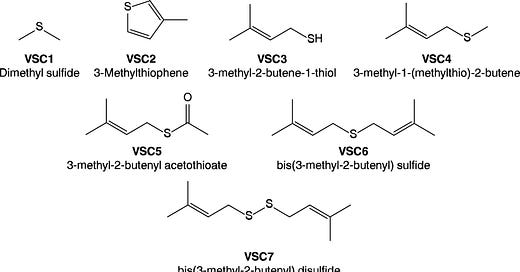
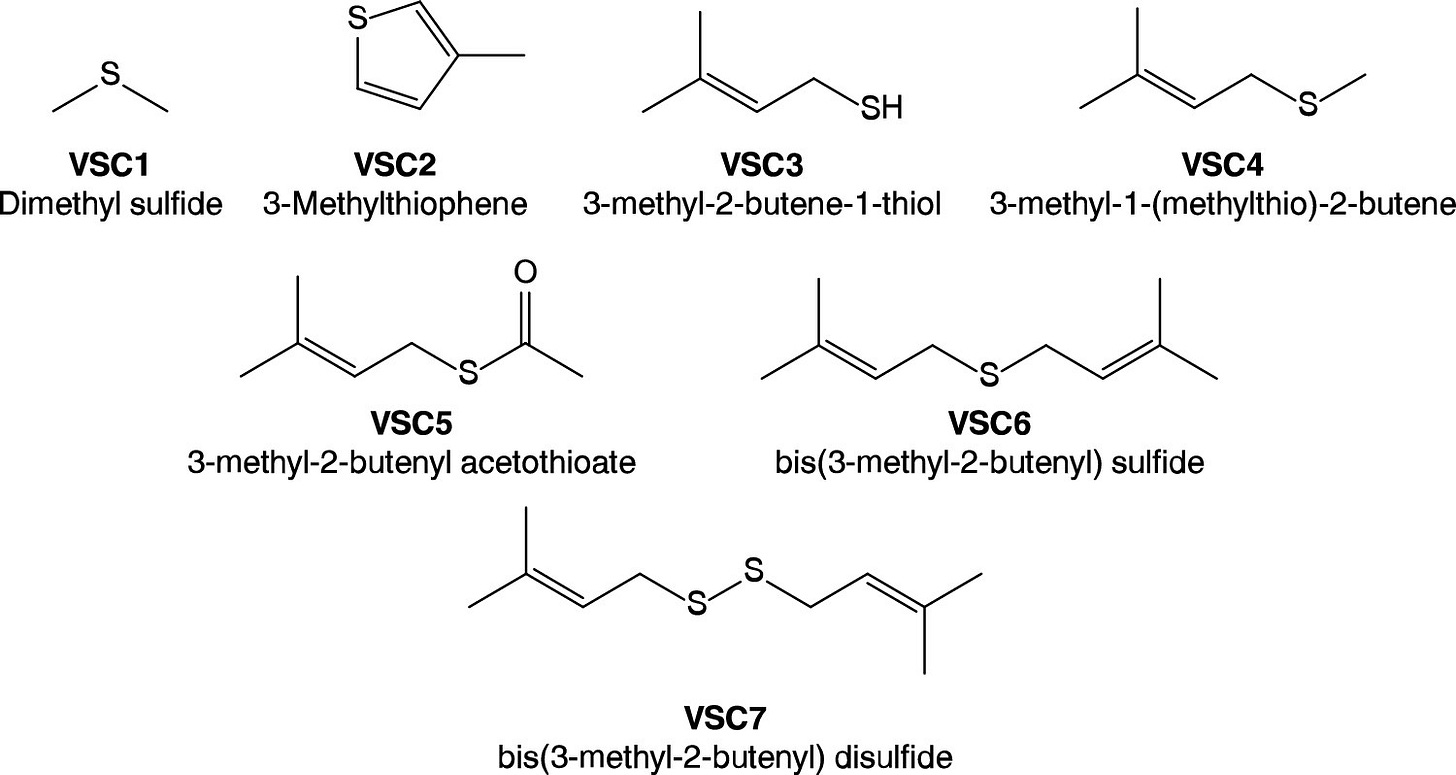
Everything changes with the publication of a new paper in the scientific journal ACS Omega (Oswald, et al., 2021). A team in Southern California led by R&D types at Abstrax Tech has identified a family of seven different sulfur-containing molecules that are the likely basis for the funkadelic ganja note of weed. They got their result with some high-powered analytic instrumentation, to wit:
a custom-built comprehensive 2-dimensional gas chromatography (GC × GC) system with three detectors operating simultaneously: A time-of-flight mass spectrometer (TOF-MS), flame ionization detector (FID), and sulfur chemiluminescence detector (SCD).
Or as that earlier bunch of SoCal coastals once sang,
Just a little deuce coupe with a flathead mill
But she’ll walk a Thunderbird like she’s standin’ still
She’s ported and relieved and she’s stroked and bored.
She’ll do a hundred and forty with the top end floored
This souped-up lab hardware allowed them to identify seven volatile sulfur compounds (VSCs) from a series of 13 cannabis cultivars. Using trained sensory panelists, they were able to characterize the aromas. They observed “a strong correlation between VSC3 concentration and the pungency of the characteristic “skunk-like” aroma of cannabis from the olfactory tests.” VSC3, mirabile dictu, turns out to be 321 MBT. The other VSCs appear to play a subordinate role in delivering the skunky note to cannabis bud.
To nail down their result, the crew “reverse engineered” the aroma, i.e., they assembled the top ten terpenes with and without VSC3 (a.k.a. 321 MBT) and had the odor judges evaluate the samples. The results?
We found that although the scent was mildly reminiscent of the flower, it did not possess the pungent, skunk-like aroma. Addition of VSC3 at approximately 1% dilution (1% VSC3 in triacetin) resulted in an immediate olfactory change that strongly emulated the scent of the flower.
Sweet!
As icing on the cake, the research team analyzed VSC concentrations in three butane hash oil concentrates and found that VSC5 (3-methyl-2-butenyl acetothioate) was the main source of pungent, skunk-like notes in that material.
Finally, as the sugar plum on top of the icing on the cake, the team grew some cannabis plants and harvested flowers at weekly intervals from early appearance to full maturity, then analyzed the samples on the Little Deuce Coupe 2-D GC setup. No VSCs were detected until week 7 and then only at low concentrations. But the VSC levels ramped up dramatically through week 10, when the samples were harvested, and increased after a week of curing. Now we know why odor complaints about cultivation facilities tend to cluster right around harvest time.
All in all a nice piece of scientific sleuthing.
We now have a much more complete picture of cannabis odor components. This could lead to the design of more realistic and compelling cannabis products. And hopefully some day, in the not too distant future, the neighbors will be able to put away their torches and pitchforks.
Iain W. H. Oswald, Marcos A. Ojeda, Ryan J. Pobanz, Kevin A. Koby, Anthony J. Buchanan, Josh Del Rosso, Mario A. Guzman, and Thomas J. Martin. (2021). Identification of a new family of prenylated volatile sulfur compounds in cannabis revealed by comprehensive two-dimensional gas chromatography. ACS Omega, published online November 12, 2021.
Felipe San-Juan, Juan Cacho, Vicente Ferreira, and Ana Escudero. (2012). 3-Methyl-2-butene-1-thiol: Identification, analysis, occurrence and sensory role of an uncommon thiol in wine. Talanta, 99: 225-231.
Full disclosure: I worked on a terpene aroma project with Abstrax Tech.